Climbing out of the abyss between animal models of Parkinson's and reality
There are so many important questions in Parkinson’s disease that we must face. The dialogue will be an important element to speed us on the path to better treatments. We sat down with Dr. Megan Duffy to pick her brain about modeling Parkinson’s disease in the laboratory and the successes and failures of techniques. We also got her take on why articles in the news so often claim a cure and fail to deliver. Finally, we discuss future directions and paths we may all take toward Parkinson’s enlightenment.
Who is Megan Duffy?
Megan Duffy, Ph.D. is a postdoctoral fellow in the Laboratory of Neurogenetics at the National Institutes of Health/National Institute on Aging. Motivated by her grandmother’s diagnosis of Parkinson’s disease in 2009, she volunteered as a research assistant in a lab studying Huntington’s disease which solidified her desire to pursue a career in research. She graduated from Indiana University in 2013 with a B.S. in Psychology and Area Certificate in Neuroscience. She attended Michigan State University, where her research investigated the role and timing of neuroinflammation in a novel model of synucleinopathy; she completed her Ph.D. in Neuroscience in 2018. Her current research interests include building better models to study the intersection of PD, genetics, and aging using induced pluripotent stem cell (iPSC) derived organoids and glial cells. Outside of the lab, she enjoys engaging with the broader PD community and helping to build bridges between patients, researchers, and physicians.
1. How much of PD can we model?
The first time this question was posed to me by a friend who has young onset Parkinson’s, it caused me to pause and think. Really think. Like most questions in neuroscience, there’s no straightforward answer; and in the case of Parkinson’s, no definitive “percentage of human Parkinson’s features” that we can model [or think we are modeling] at a given time.
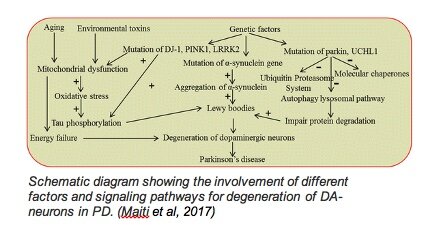
In the past few years, there has been a shift in thinking about how we define PD, and neurodegenerative diseases in general, given the variability in age of onset, genetic contributions, symptoms, and speed of progression. I heard once, “If you’ve met one person with Parkinson’s, you know one person with Parkinson’s;” in other words, PD manifests differently in each person, and putting all of those variations into one box may limit how we think about and study PD both preclinically and in clinical trials. In science, we tend to put mechanisms of a disease or phenomenon in a box; studying how disrupting one gene or protein or cell type results in an observable phenotype. While it certainly makes drawing conclusions easier and more specific, we need to remember that there are often many processes that go awry in disease and then feed into or have downstream effects on each other (see diagram above) collectively, resulting in neurodegeneration.
The short answer? There isn’t one. However, I hope that we can shift our language from “modeling Parkinson’s” to “modeling X aspect or feature of Parkinson’s.” As discussed below, humans are the only species that naturally develops PD. Being more honest and specific about what element of PD we are studying, in what model, and why that benefits the science community will likely lead to more accurate translation of findings for the public. Everyone wins.
2. “What’s is the best model out there to study
Parkinson’s?”
A familiar and regularly debated question. And, you guessed it, there’s no singular answer. A quote I saw in a presentation, which I refer to often, sums it up well:
“All models are wrong, but some may be useful…So since all models are wrong, it is very important to know what to worry about… the question you need to ask is not ‘Is the model true?’ (it never is) but ‘Is the model good enough for this particular application?’”
-Statistician George Box, 1976
A few years later…
“Modelling in science remains, partly at least, an art. Some principles do exist, however, to guide the modeller. The first is that all models are wrong; some, though, are better than others, and we can search for the better ones. At the same time, we must recognize that eternal truth is not within our grasp.”
-Peter McCullagh and John Nelder, 1983
The best model for a given study is whatever model is appropriate for the question you are asking. If we want to study a potential neuroprotective agent, we wouldn’t use a model in which neurodegeneration happens rapidly (several days to weeks), as in humans it likely takes decades. If we want to study aggregation of alpha-synuclein, we wouldn’t use a neurotoxin model which has little to no alpha-synuclein pathology, etc…etc…
Every model used to study PD has merits and limitations and is an approximation to the human condition, because there are obvious limitations when studying humans. For scientists: pick the most appropriate model for your question and be transparent about what it can and cannot tell us. For the patient community: don’t be afraid to ask scientists about the models they use and how it affects the conclusions drawn from them!
3. We’ve “cured” Parkinson’s in mice hundreds
of times, but no disease-slowing therapy has
successfully been translated to humans. Why?
It’s important to keep in mind that Parkinson’s is a complex human-specific condition with many contributing factors. This can make interpretations drawn from models somewhat limited. Some notable differences to keep in mind:
· Aging and longevity – Quite simply, humans live longer than the species we use to model diseases in the lab. Aging remains the largest risk factor for developing PD. The human lifespan is >35x that of a laboratory rodent (~79 years vs. 2 years), leaving more time for normal homeostatic processes to go awry and their downstream consequences to produce symptoms.
· Brain complexity
· Neurodevelopmental timeline
· Genetics – First and most obvious, we don’t have rodent, worm, or non-human primate genomes. Second, genes are not always disease-causing by themselves. Many mutations that have no effect individually can act synergistically with each other and/or environmental and lifestyle factors which collectively cause disease. There are far more possible combinations than we can systematically model.
· Environment and Lifestyle– We don’t develop as a flat layer of cells in isolation on a plastic dish, nor are we animals that all live in the same controlled environment, eat the same diet, get the same amount of sleep, or are exposed to the same external environment.
· Lack of biomarkers for early diagnosis— The underlying pathophysiology of Parkinson’s likely begins decades before symptoms become evident. On average, the loss of dopaminergic fibers is nearly complete by 4 years post-diagnosis (Kordower et al., 2013), very much limiting the timeframe when a neuroprotective therapy could have a chance of succeeding i.e., you cannot protect what no longer exists. Thus the earlier the diagnosis, the better. In contrast, in the lab, neuroprotective therapies are administered in models prior to any neurodegeneration. This key difference in timing is likely a major contributor to the “translational abyss” between promising therapeutic candidates preclinically and therapies that succeed in clinical trials, actually making their way to patients. Luckily, there has been significant emphasis and investment in discovering biomarkers that may help us predict and/or diagnose disease earlier.
4. News headlines about science are often
sensationalized or over-hyped, which can lead to
false hope, unrealistic expectations, and [if the
results don’t pan out] disappointment. What are
some questions to ask myself and what are some
red flags to be on the lookout for when reading
about science in the new?
· Sensationalized headlines: Unfortunately, success sells and negative results (which are just as important!) do not.
· Buzzwords and phrases: “cured,” “reversed,” “discovery,” “groundbreaking.”
· Does the headline pull at your heartstrings?
· Remember, humans are the only species who develop PD naturally. Mice do not. A cure for “PD” in mice ≠ PD in humans.
· Do they link to the original, peer-reviewed article?
· Does the headline specify “in humans?” Many articles claiming discovery of a “cure” or “groundbreaking new treatment” are referencing preclinical work in cells or animal models, but it’s not always explicitly stated.
Now, there are also many well-informed and well-translated pieces in the mainstream media that may use certain buzzwords, and justifiably so (think COVID-19 vaccine development). A combination of the above red flags can help you to distinguish reality vs. hype and allow you to temper your expectations accordingly.
5. Scientific papers are often full of jargon that
can be difficult to follow. Where can I find
information about the science of PD broken down
in an accessible way?
· The National Institutes of Health provides science-based, lay-friendly information on Parkinson’s disease. You can find information on the following websites:
o The National Institute on Aging Parkinson’s information: https://www.nia.nih.gov/health/parkinsons-disease
o The National Institute of Neurological Disorders and Stroke
https://www.ninds.nih.gov/Disorders/All-Disorders/Parkinsons-Disease-Information-Page
Additionally, blogs can act as another resource. Below are just a few that can provide helpful information.
· The Science of Parkinson’s: Plain English information about the research being conducted on PD.
Author: Simon Stott, Ph.D., Deputy Director of Research at the Cure Parkinson’s Trust.
This blog covers all things PD from clinical trials to top PD news, basic biology, and recent papers.
· Tomorrow Edition Interviews
Author: Benjamin Stecher, Patient Advocate and Consultant (and coauthor of Brain Fables)
Motivated by his own PD diagnosis at age 29, Ben has traveled the world meeting and interviewing physicians and scientists in all facets of PD treatment and research, which are recorded and transcribed on his blog, Tomorrow Edition.
· Journey with Parkinson’s "Where Life Meets Parkinson's." A blog for Parkinson's education, research advances, treatment strategies, and personal reflection.
Author: Frank Church, Ph.D., Professor in Departments of Pathology and Laboratory Medicine, Pharmacology, and Medicine at University of North Carolina Chapel Hill School of Medicine and person living with Parkinson’s disease.
This blog combines scientific expertise with personal experience to break down complex concepts in an informative but accessible way.
These are just a few resources that may be of interest to you.
· If you find a scientific paper that piques your interest and you’d like to know more, you can email the first or last author. A response isn’t guaranteed, but generally scientists enjoy and are open to discussing their work with patient communities.
6. Despite the complexity of PD and limitations
of what models can tell us, what gives me hope
looking forward?
· Open science initiatives – making science more transparent, reproducible, standardized, collaborative, and accessible (AMP PD, ASAP Initiative, protocols.io)
· Large scale biobanking, characterization, and integration of *omics & functional data from patient cell lines and genome-engineered controls (FoundinPD, iNDI)
· Increasing dialogue between PwP, scientists, and physicians
· Technology advancing at a rapid pace. For example, the Human Genome Project took a little over ten years. Just published this week: a case report of an infant admitted to the hospital, his entire genome sequenced, causative defect identified, and treatment started in less than 36 hours.
References:
Maiti, Panchanan et al. “Current understanding of the molecular mechanisms in Parkinson's disease: Targets for potential treatments.” Translational neurodegeneration vol. 6 28. 25 Oct. 2017, doi:10.1186/s40035-017-0099-z
Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, Halliday GM, Bartus RT. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain. 2013 Aug;136(Pt 8):2419-31. doi: 10.1093/brain/awt192. PMID: 23884810; PMCID: PMC3722357.
Robledo, Israel, and Joseph Jankovic. “Media hype: Patient and scientific perspectives on misleading medical news.” Movement disorders : official journal of the Movement Disorder Society vol. 32,9 (2017): 1319-1323. doi:10.1002/mds.26993
Owen MJ, Niemi AK, Dimmock DP, Speziale M, Nespeca M, Chau KK, Van Der Kraan L, Wright MS, Hansen C, Veeraraghavan N, Ding Y, Lenberg J, Chowdhury S, Hobbs CA, Batalov S, Zhu Z, Nahas SA, Gilmer S, Knight G, Lefebvre S, Reynders J, Defay T, Weir J, Thomson VS, Fraser L, Lajoie BR, McPhail TK, Mehtalia SS, Kunard CM, Hall KP, Kingsmore SF. Rapid Sequencing-Based Diagnosis of Thiamine Metabolism Dysfunction Syndrome. N Engl J Med. 2021 Jun 3;384(22):2159-2161. doi: 10.1056/NEJMc2100365. PMID: 34077649.
**All views and opinions expressed are my own and do not reflect those of NIH
To read more books and articles by Michael S. Okun MD check on Twitter @MichaelOkun and these websites with blogs and information on his books and http://parkinsonsecrets.com/ #Livingwith Parkinson’s #EndingPD #Parkinsonsecrets #LessonsFromTheBedside
He also serves as the Medical Advisor for the Parkinson’s Foundation.
To see more on Dr. Indu Subramanian she does live interviews of experts in Parkinson’s for the PMD Alliance.
The blog artist is Jonny Acheson.