Gene Therapy for Brain Diseases: A Path for DYT-1 Dystonia
Sciworthy.com (picture) which has great images and a great summary website of this topic!
Gene Therapy for DYT-1 Dystonia and other diseases?
We sat down with one the experts on gene therapy for DYT-1 dystonia and other central nervous system diseases and we picked his brain on this topic. The interview was fascinating!
Who is Edgar Rodriguez?
Dr. Edgardo (Edgar) Rodríguez-Lebrón has 20 years of experience in the gene therapy space with a focus on diseases of the nervous system. He has engineered and developed gene-based therapies to prevent or reverse neurodegenerative processes caused by the accumulation of toxic mutant proteins. He is currently Scientific Advisor to Tyler's Hope, Chief Science Officer of Andante Biologics and a co-founder of early-stage biotech companies developing molecular therapies for CNS diseases. He holds an Adjunct Faculty appointment at the University of Florida where he trained as a graduate student. Go Gators!
Often, diseases that affect the brain are caused by genetic mutations that prevent the normal function of a gene. In other cases, genetic mutations can cause a gene to ‘gain’ a new function that is toxic to the brain.
Gene therapies are a class of medicines designed to specifically (i) replace a missing genetic function, (ii) block a ‘gained’ toxic genetic function, or (iii) limit or enhance cellular pathways to support overall brain function.
What is a viral-based gene therapy?
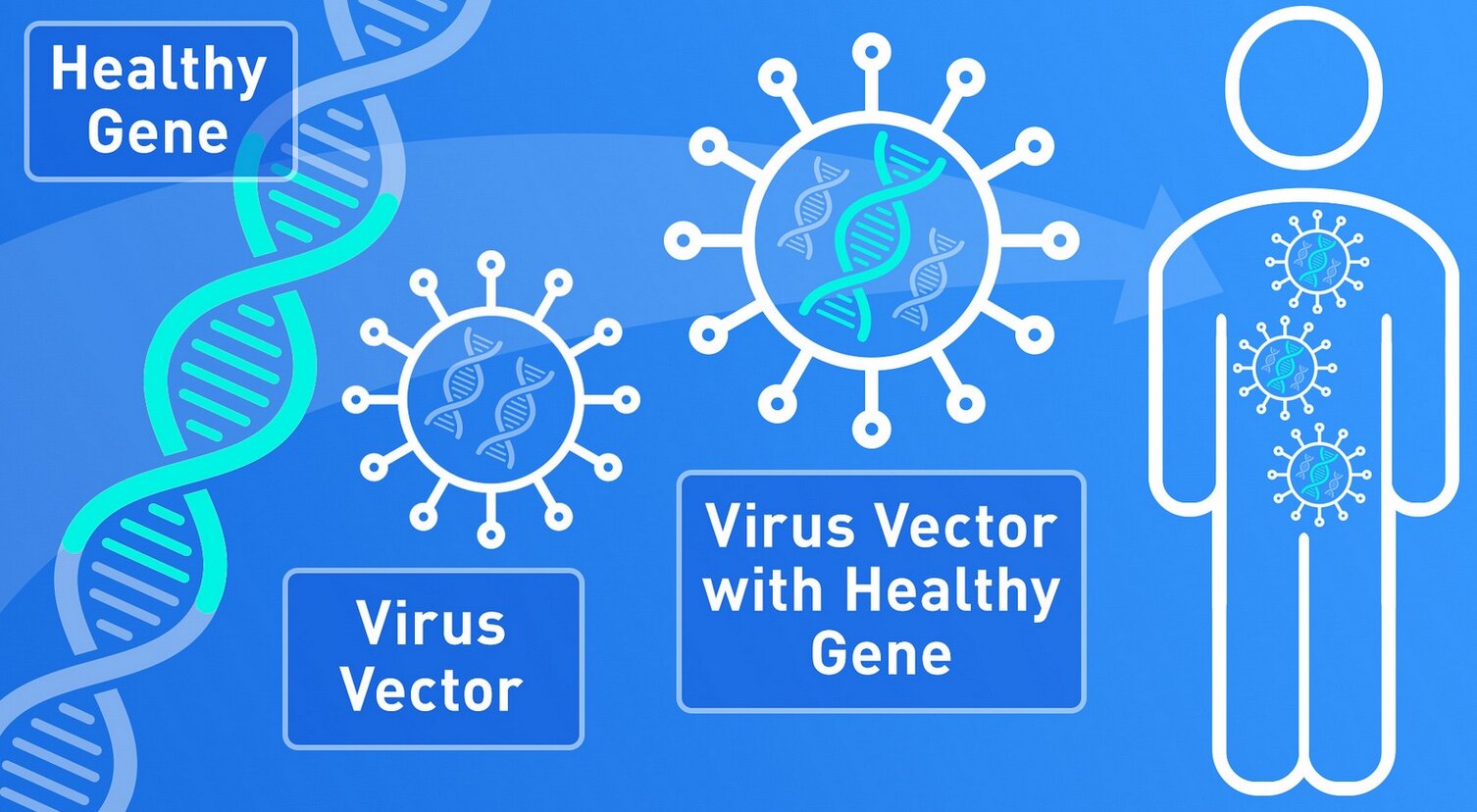
It is a type of gene therapy that uses a virus as a vector, think a ‘shuttle’ or ‘delivery truck’, to introduce genetic cargo into cells. The genetic cargo is designed to replace a missing gene, or suppress the function of a toxic gene, or modify cellular pathways to support normal tissue function.
Viruses are fully optimized genetic machines capable of penetrating into cells and delivering genetic material with high efficiency. One virus capable of delivering genetic material to neurons and other cells in the brain in a safe and effective fashion is the adeno-associated virus (AAV).
AAV is a naturally occurring virus that has not been connected to any known human disease or pathology. When used as a gene therapy vector, 95% of the AAV’s natural genetic sequence is removed and replaced with the therapeutic genetic cargo.
AAV vectors used in gene therapy can enter the cell and deliver therapeutic DNA but are incapable of replicating and cannot sustain a normal virus infection cycle.
What makes AAV a desirable vector for CNS-targeted gene therapies?
AAV is desirable because:
AAV has evolved a natural ability to enter human neurons and effectively deliver genetic material to their nucleus.
The genetic material delivered by AAV vectors does not become incorporated into human DNA, i.e. the human genome, and instead remains as a stable, independent molecule within the nuclear compartment of the cell. This drastically lowers the risks associated with randomly inserting pieces of genetic material into the human genome.
There are many variants or ‘flavors’ of AAV that differ in their protein capsid shell (i.e. the outer covering that shields and encapsulates the genetic material). This diversity allows scientists to select particular AAV vector variants to achieve effective delivery of genetic material into different cells in our bodies, including neurons.
For example, some AAV vector variants can be infused into the circulatory system, cross into the brain, and deliver genetic material to neurons in the central nervous system.
What are some recent major developments in AAV CNS gene therapy?
Recent developments include:
The most important recent development was the approval of 2 different AAV-based gene therapy drug products by the FDA. One of these, Zolgensma made by Novartis, is an AAV gene therapy for the treatment of Spinal Muscular Atrophy.
More than 40 clinical studies have investigated or are investigating the therapeutic potential of AAV-based gene therapies in human CNS diseases.
Artificial intelligence and machine learning algorithms are now being employed to redesign and engineer the AAV protein capsid shells to generate novel variants with more effective, targeted gene delivery properties.
Large-scale manufacturing of clinical-grade AAV vectors is becoming streamlined.
What are some of the major hurdles still being faced by AAV-based gene therapies?
Hurdles include:
AAV vectors are live viruses that can be recognized by the immune system as ‘foreign’ agents.
More than half of us have already developed some level of ‘immunity’ to naturally occurring variants of AAV that exist in the population.
This ‘pre-existing’ immunity to AAV needs to be overcome for AAV gene therapy vectors to effectively reach their target cells and deliver the therapeutic genetic material before being neutralized by the immune system.
To address this challenge, clinicians are testing immunosuppressive drug cocktails that could temporarily put the immune surveillance system ‘to sleep’ while the AAV gene therapy delivers the genetic cargo.
At the same time, scientists continue to re-engineer the AAV vector capsid shell to make it be like a stealth genetic delivery shuttle system.
How can an AAV-based gene therapy be used in DYT1 dystonia?
Applications to DYT-1:
DYT1 dystonia is primarily caused by the inheritance of mutations in the TOR1A gene.
It remains unclear exactly how mutations in TOR1A lead to dystonia. However, multiple studies suggest that the primary TOR1A mutation, p.302/p.303delE, destabilizes the protein, causing a partial loss of gene function.
The matter is further complicated by the fact that the TOR1A protein likes to self-associate. It appears that in DYT1, when the mutant form of TOR1A associates with the normal form of TOR1A, it impairs the function of the normal TOR1A protein.
Based on this important knowledge, researchers are designing and testing AAV-based therapies to (1) enhance the function of the normal TOR1A protein, (2) selectively block expression of the mutant TOR1A protein or (3) enhancethe function of the normal TOR1A protein and simultaneously block expression of the mutant form of TOR1A.
What is on the horizon for AAV-based gene therapies as it applies to DYT1?
On the horizon:
Identification of the neurons and brain regions that are primarily affected in DYT1. This will help guide AAV-based gene therapies by providing ‘the right zip code’.
Engineering and discovery of new AAV capsid variants that can more broadly target affected brain regions in the DYT1 brain.
Deployment of genetic cargo that can facilitate direct and precise ‘editing’ of the TOR1A genomic sequence to replace disease-causing mutated sequences in the TOR1A gene with non-disease causing sequences.
To read more books and articles by Michael S. Okun MD check Twitter @MichaelOkun and these websites with blogs and information on his blogs and books Dystonia Tips and Research @TylersHope #TylersHope #Dystoniasucks